Overview: Secure storage & accelerated research
Statement of Purpose
What is the Ontario Tumour Bank?
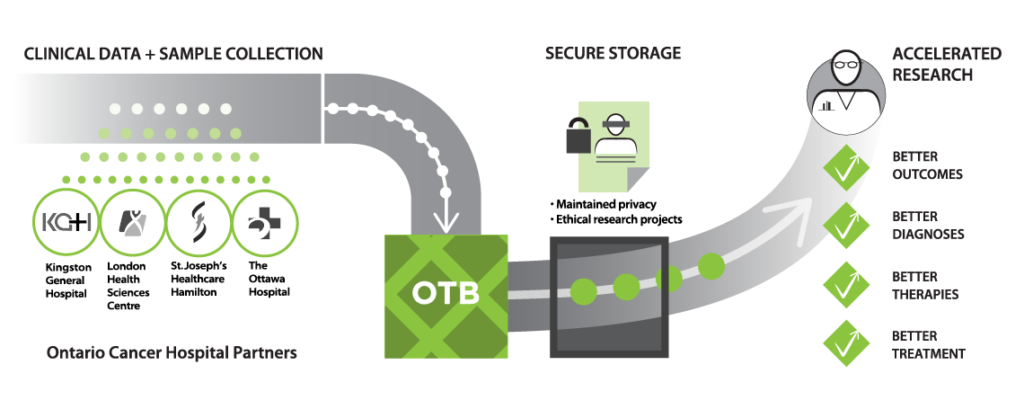
The Ontario Tumour Bank (OTB) is a provincial biorepository that collects tissue, blood and Personal Health Information (PHI) from consenting volunteer participants and was established in response to a growing need for a provincial tissue and data bank to support cancer research. The outcomes of the research studies are expected to contribute to the provision of health care for cancer patients by providing information that may lead to an increased understanding of the disease, and the development of new diagnostic tools and therapies.
OTB is one of several programs of the Ontario Institute for Cancer Research (OICR), a not-for-profit corporation that is funded by the Government of Ontario. Under section 39(1)(c) of the Personal Health Information Protection Act, 2004 (PHIPA), the Ministry of Health and Long-Term Care has prescribed the OICR in respect of OTB as a registry of personal health information. In order to fulfill this role, OICR has implemented policies, procedures and practices to protect the privacy of individuals whose personal health information it receives and to maintain the confidentiality of that information.
Tissue, blood, and associated PHI/data are collected from participants who have provided informed consent at one of three (3) collection centres in Ontario: London Health Sciences Centre (London), St. Joseph’s Healthcare Hamilton (Hamilton) and The Ottawa Hospital (Ottawa). OTB makes this source of high quality samples and data available to cancer researchers worldwide. Researchers may request samples and de-identified clinical data from OTB by submitting an application form with a valid research ethics board approval letter for their research study. A Material Transfer Agreement (MTA) must also be signed. OTB must review and approve the application before any samples and de-identified clinical data are provided to researchers. OTB biospecimens and data are a foundational resource for scientists engaged in translational research who are developing diagnostic tools and new drug therapies, for the enhancement of patient care.
Where does OTB get PHI?
For patients who have consented to participate, OTB collects data, including PHI, from patient health records at participating hospital collection centres. These centres are classified under the Personal Health Information Protection Act, 2004 (PHIPA) as ‘health information custodians’ (HICs). OTB’s data may be linked to additional health information from Ontario Health/Cancer Care Ontario (CCO). Ontario Health/CCO is a provincial agency that is responsible for continually improving cancer services and is the government’s cancer advisor; Ontario Health/CCO is designated as a ‘prescribed entity’ under PHIPA.
What type of PHI does OTB collect?
PHI collected from the patient health record includes demographic information (e.g. age, sex), details of the cancer diagnosis (e.g. type of cancer, grade, stage), treatment details, patient and family history of cancer, risk factors, and outcome information concerning the progression of the disease or the disease-free status.
This information may be supplemented with data from Ontario Health/CCO’s Ontario Cancer Registry, which includes additional outcome, diagnosis and treatment details. OTB also collects direct identifiers (e.g., name, date of birth, medical record number) to enable longitudinal and comprehensive data collection.
PHI that is collected by OTB, including information provided by Ontario Health/CCO, is de-identified before it is used for research purposes. This means that information that could directly identify an individual is removed from the data and that researchers do not receive this information.
How does OTB use PHI?
OTB does not perform research and therefore does not use, disclose or retain PHI or de-identified clinical data for its own research purposes. The use, disclosure and retention of data that is collected by OTB is for the stated purpose, which is to maintain a high quality registry of biospecimens and accompanying clinical data for the facilitation of cancer research.
To whom does OTB disclose PHI?
OTB discloses de-identified clinical data and samples to researchers for the conduct of cancer research. OTB does not permit PHI (direct identifying information) to be disclosed for research under any circumstances. The de-identified data and samples are disclosed to academic and industry-based researchers whose research proposals (applications) must be accompanied by a research ethics board approval letter. OTB also reviews and approves the application and ensures that a Material Transfer Agreement (MTA) must be signed between OICR and the researcher before any samples or data are provided to the researcher. This agreement includes provisions to ensure that the researcher will maintain the confidentiality of the data and will not attempt to re-identify any participants. Researchers who have met these criteria can then use the samples and de-identified clinical data to conduct their research. The outcomes of these research studies are expected to contribute to the provision of health care for cancer patients by providing information that may lead to an increased understanding of the disease, and the development of new diagnostic tools and therapies.
OTB is permitted to disclose PHI for non-research purposes in specified circumstances as permitted by PHIPA, 2004 and its regulation. For example, OTB may disclose PHI to Ontario Health/Cancer Care Ontario (CCO) for the purpose of linking the PHI collected at the participating collection centres (hospitals) to additional information that is in the custody and control of Ontario/Health CCO (e.g., data within the Ontario Cancer Registry, the provincial cancer registry). This disclosure is permitted under s. 49 of the Act. OTB will disclose PHI only if de-identified or aggregate data will not serve the purpose.
How can I get access to my information?
Persons requesting access to their information will be directed to the health information custodian from where the information was collected.
Whom can I speak to if I have questions about OTB’s information practices and privacy program?
Questions, concerns or complaints related to OTB’s information practices or privacy program should be submitted in writing and addressed to the OICR Privacy Officer, as set out below. All inquiries will be dealt with in a timely fashion.
Ontario Institute for Cancer Research
Attn: Privacy Officer
MaRS Centre
661 University Avenue, Suite 510
Toronto, Ontario, Canada
M5G 0A3
Phone: 416-673-8574
Email: privacy@oicr.on.ca
Any complaints regarding OTB’s compliance with PHIPA, 2004 and its regulation may be directed to the Information and Privacy Commissioner of Ontario:
Information and Privacy Commissioner/Ontario
2 Bloor Street East, Suite 1400
Toronto, Ontario, Canada
M4W 1A8
Web: www.ipc.on.ca
Telephone: 416-326-3333 or 1-800-387-0073.

